




Coming Soon
Coming Soon
Coming Soon
Test Kits
Click on Any Button
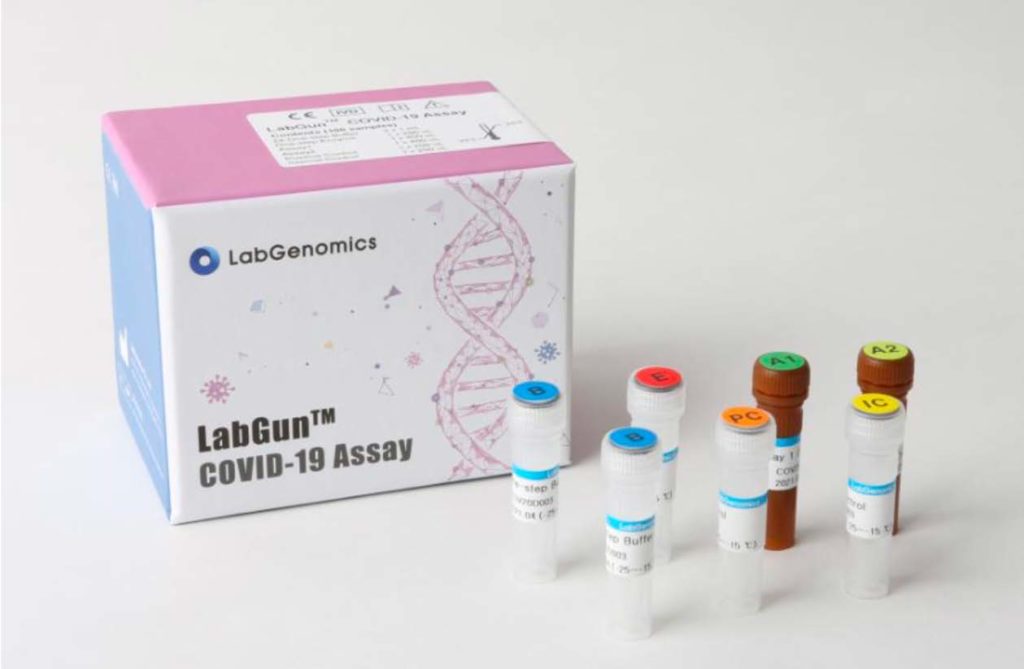
LabGenomics LabGun Covid-19 Assay
The LabGunTM COVID-19 Assay PCR Kit is intended for the
qualitative detection of Coronavirus disease 2019 (COVID-19)
strain SARS-CoV-2, in patients that meet the clinical criteria for
COVID-19 (e.g. fever, cough, shortness of breath) in the lower
respiratory tract or upper respiratory tract.
The kits are approved by the US FDA and the EU. Trials on the
kits have yielded sensitivity and specificity in excess of 99%.
Tests run on the Real-time PCR CFX96TM (Bio-Rad), Applied
BiosystemsTM 7500 Real-time PCR Instrument system (Thermo
Fisher Scientific), or Applied BiosystemsTM 7500 Fast Real-time
PCR Instrument system (Thermo Fisher Scientific)
Each kit contains sufficient supplies for 100 tests.
In March, 2020 LabGenomics supplied 500,000 to the State of
Maryland through a personal purchase negotiated by the state’s
Governor.
Price: Quote
Origin: Republic of Korea
Lead time: 21 days
Shipping: FOB Korea
Production: 10 mm tests / mo


April 29, 2020
Myoung Shin Kim, LabGenomics Co., Ltd., Gyeonggi Bio Center #1204,147 Gwanggyo-ro, Yeongtong-gu, Suwon-si, KR Gyeonggi-do, Republic of Korea
Device: LabGun COVID-19 RT-PCR Kit
Company: LabGenomics Co., Ltd.
Indication: Qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal, or oropharyngeal, anterior nasal and mid-turbinate nasal swabs, as well as nasopharyngeal wash/aspirate or nasal aspirate specimens and sputum, from individuals who are
suspected of COVID-19 by their healthcare provider. Emergency use of this test is limited to authorized laboratories. Authorized Laboratories: Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests.
Dear Myoung Shin Kim:
This letter is in response to your1 request that the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for emergency use of your product,2 pursuant to Section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. §360bbb-3).
On February 4, 2020, pursuant to Section 564(b)(1)(C) of the Act, the Secretary of the
Department of Health and Human Services (HHS) determined that there is a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad, and that involves the virus that causes COVID-19. Pursuant to Section 564 of the Act, and on the basis of such determination, the Secretary of HHS then declared that circumstances exist justifying the authorization of emergency use of in 1 For ease of reference, this letter will use the term “you” and related terms to refer to LabGenomics Co., Ltd. 2 For ease of reference, this letter will use the term “your product” to refer to the LabGun COVID-19 RT-PCR Kit
used for the indication identified above.
Page 2 – Myoung Shin Kim, LabGenomics Co., Ltd.
vitro diagnostics for detection and/or diagnosis of the virus that causes COVID-19 subject to the terms of any authorization issued under Section 564(a) of the Act.3
Having concluded that the criteria for issuance of this authorization under Section 564(c) of the Act are met, I am authorizing the emergency use of your product, described in the Scope of Authorization of this letter (Section II), subject to the terms of this authorization.
I. Criteria for Issuance of Authorization
I have concluded that the emergency use of your product meets the criteria for issuance of an authorization under Section 564(c) of the Act, because I have concluded that:
1. The SARS-CoV-2 can cause a serious or life-threatening disease or condition,
including severe respiratory illness, to humans infected by this virus;
2. Based on the totality of scientific evidence available to FDA, it is reasonable to believe
that your product may be effective in diagnosing COVID-19, and that the known and
potential benefits of your product when used for diagnosing COVID-19, outweigh the
known and potential risks of your product; and,
3. There is no adequate, approved, and available alternative to the emergency use of your product.
4 II. Scope of Authorization
I have concluded, pursuant to Section 564(d)(1) of the Act, that the scope of this authorization is limited to the indication above.
Authorized Product Details Your product is a qualitative test for the detection of nucleic acid from SARS-CoV-2 in nasopharyngeal, or oropharyngeal, anterior nasal and mid-turbinate nasal swabs, as well as nasopharyngeal wash/aspirate or nasal aspirate specimens and sputum, from individuals who are suspected of COVID-19 by their healthcare provider. The SARS-CoV-2 nucleic acid is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 nucleic acid; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status.
Positive results do not rule out bacterial infection or co-infection with other viruses.
To use your product, SARS-CoV-2 nucleic acid is first extracted, isolated and purified from
nasopharyngeal, or oropharyngeal, anterior nasal and mid-turbinate nasal swabs, as well as nasopharyngeal wash/aspirate or nasal aspirate specimens and sputum. The purified nucleic acid is then reverse transcribed into cDNA followed by PCR amplification and detection using an 3 U.S. Department of Health and Human Services, Determination of a Public Health Emergency and Declaration that Circumstances Exist Justifying Authorizations Pursuant to Section 564(b) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3. 85 FR 7316 (February 7, 2020).
4 No other criteria of issuance have been prescribed by regulation under Section 564(c)(4) of the Act.
Page 3 – Myoung Shin Kim, LabGenomics Co., Ltd.
authorized real-time (RT) PCR instrument. The LabGun COVID-19 RT-PCR Kit includes the
following materials or other authorized materials: 2X One-step buffer, One-step enzyme, Assay
1 (RdRp gene), Assay 2 ( E gene), MS2 Internal control and Positive control.
Your product requires the following control materials, or other authorized control materials, that are processed in the same way as the specimens and are required to be included with each batch of specimens tested with your product. All controls listed below must generate expected results in order for a test to be considered valid, as outlined in the Instructions for Use:
* Internal Control (IC)- MS2 phage IC should be added to each specimen prior to
extraction, and controls for specimen quality and demonstrates that nucleic acid was
generated by the extraction process.
* Positive Control – contains cloned plasmid DNAs of the RdRp and E gene genomic
regions targeted by the kit. The positive control is used to monitor for failures of
PCR reagents and reaction conditions.
* Negative Control – RNase-free water (Diethyl pyrocarbonate (DEPC)-water) used to
monitor non-specific amplification, cross-contamination during experimental setup,
and nucleic acid contamination of reagents.
Your product also requires the use of additional authorized materials and authorized ancillary reagents that are not included with your product and are described in the Instructions for Use.
The above described product, when labeled consistently with the labeling authorized by FDA, entitled “Instructions for LabGun COVID-19 RT-PCR Kit” (available at
https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use authorizations), which may be revised in consultation with, and with concurrence of, the Division of Microbiology Devices (DMD)/Office of Health Technology 7 Office of In Vitro Diagnostics and Radiological Health (OHT7-OIR)/Office of Product Evaluation and Quality (OPEQ)/Center for Devices and Radiological Health (CDRH), is authorized to be distributed to and used by authorized laboratories under this EUA, despite the fact that it does not meet certain requirements otherwise required by applicable federal law.
Your product is authorized to be accompanied by the following product-specific information pertaining to the emergency use, which is required to be made available to healthcare providers and patients:
* Fact Sheet for Healthcare Providers: LabGun COVID-19 RT-PCR Kit
* Fact Sheet for Patients: LabGun COVID-19 RT-PCR Kit
I have concluded, pursuant to Section 564(d)(2) of the Act, that it is reasonable to believe that the known and potential benefits of your authorized product, when used for the qualitative detection of SARS-CoV-2 and used consistently with the Scope of Authorization of this letter (Section II), outweigh the known and potential risks of your product.
Page 4 – Myoung Shin Kim, LabGenomics Co., Ltd.
I have concluded, pursuant to Section 564(d)(3) of the Act, based on the totality of scientific evidence available to FDA, that it is reasonable to believe that your product may be effective for the indication above, when used consistently with the Scope of Authorization of this letter (Section II), pursuant to Section 564(c)(2)(A) of the Act.
FDA has reviewed the scientific information available to FDA, including the information
supporting the conclusions described in Section I above, and concludes that your product (as described in the Scope of Authorization of this letter (Section II)) meets the criteria set forth in Section 564(c) of the Act concerning safety and potential effectiveness.
The emergency use of your product under this EUA must be consistent with, and may not exceed, the terms of this letter, including the Scope of Authorization (Section II) and the Conditions of Authorization (Section IV). Subject to the terms of this EUA and under the circumstances set forth in the Secretary of HHS’s determination under Section 564(b)(1)(C) described above and the Secretary of HHS’s corresponding declaration under Section 564(b)(1), your product is authorized for the indication above.
III. Waiver of Certain Requirements I am waiving the following requirements for your product during the duration of this EUA:
Current good manufacturing practice requirements, including the quality system
requirements under 21 CFR Part 820 with respect to the design, manufacture,
packaging, labeling, storage, and distribution of your product.
IV. Conditions of Authorization
Pursuant to Section 564(e) of the Act, I am establishing the following conditions on this
authorization:
LabGenomics Co., Ltd. (You) and Authorized Distributor(s)5
A. Your product must comply with the following labeling requirements under FDAregulations: the intended use statement (21 CFR 809.10(a)(2), (b)(2)); adequate directions for use (21 U.S.C. 352(f)), (21 CFR 809.10(b)(5), (7), and (8)); any appropriate limitations on the use of the device including information required under 21 CFR 809.10(a)(4); and any available information regarding performance of the device, including requirements under 21 CFR 809.10(b)(12).
B. You and authorized distributor(s) will make your product available with the authorized
labeling to authorized laboratories. You may request changes to the authorized
labeling. Such requests will be made in consultation with, and require concurrence of,
DMD/OHT7-OIR/OPEQ/CDRH.
C. You and authorized distributor(s) will provide to authorized laboratories the Fact Sheet
for Healthcare Providers and the authorized Fact Sheet for Patients. You may request
5 “Authorized Distributor(s)” are identified by you, LabGenomics Co., Ltd., in your EUA submission as an entity allowed to distribute your device.
Page 5 – Myoung Shin Kim, LabGenomics Co., Ltd.
changes to the authorized Fact Sheets. Such requests will be made in consultation
with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
D. You and authorized distributor(s) will make available on your website(s) the Fact
Sheet for Healthcare Providers and the Fact Sheet for Patients.
E. You and authorized distributor(s) will inform authorized laboratories and relevant
public health authorities of this EUA, including the terms and conditions herein, and
any updates made to your product, authorized labeling and authorized Fact Sheets.
F. Through a process of inventory control, you and authorized distributor(s) will maintain
records of the authorized laboratories to which they distribute the test and number of tests they distribute.
G. You and authorized distributor(s) will collect information on the performance of your
product. You will report to FDA any suspected occurrence of false positive and false
negative results and significant deviations from the established performance
characteristics of the product of which you become aware.
H. You and authorized distributor(s) are authorized to make available additional
information relating to the emergency use of your product that is consistent with, and
does not exceed, the terms of this letter of authorization.
LabGenomics Co., Ltd. (You)
I. You will notify FDA of any authorized distributor(s) of your product, including the
name, address, and phone number of any authorized distributor(s).
J. You will provide authorized distributor(s) with a copy of this EUA and communicate to
authorized distributor(s) any subsequent amendments that might be made to this EUA
and its authorized accompanying materials (e.g., Fact Sheets).
K. You may request to make available additional authorized labeling and fact sheets specific to an authorized distributor. Such additional labeling and fact sheets may use another name for the product, but otherwise must be consistent with the authorized labeling, and not exceed the terms of authorization of this letter. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH
L. You may request changes to the Scope of Authorization (Section II in this letter) of your
product. Such requests will be made in consultation with DMD/OHT7-OIR/OPEQ/CDRH, and require concurrence of, Office of Counterterrorism and Emerging Threats (OCET)/Office of the Chief Scientist (OCS)/Office of the Commissioner (OC) and DMD/OHT7-OIR/OPEQ/CDRH.
Page 6 – Myoung Shin Kim, LabGenomics Co., Ltd.
M. You may request the addition of other instruments and associated software for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
N. You may request the addition of other extraction methods for use with your product.
Such requests will be made in consultation with, and require concurrence of,
DMD/OHT7-OIR/OPEQ/CDRH.
O. You may request the addition of other specimen types for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
P. You may request the addition and/or substitution of primers or probes for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
Q. You may request the addition and/or substitution of control materials for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
R. You may request the addition and/or substitution of other ancillary reagents and
materials for use with your product. Such requests will be made in consultation with,
and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
S. You will evaluate the analytical limit of detection and assess traceability6 of your
product with any FDA-recommended reference material(s). After submission to FDA
and DMD/OHT7-OIR/OPEQ/CDRH’s review of and concurrence with the data, you
will update labeling to reflect the additional testing. Such labeling updates will be
made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
T. You will track adverse events, including any occurrence of false results and report to
FDA under 21 CFR Part 803. Authorized Laboratories
U. Authorized laboratories using your product will include with test result reports, all
authorized Fact Sheets. Under exigent circumstances, other appropriate methods for
disseminating these Fact Sheets may be used, which may include mass media.
V. Authorized laboratories using your product will use your product as outlined in the
Instructions for Use. Deviations from the authorized procedures, including the
authorized instruments, authorized extraction methods, authorized clinical specimen
types, authorized control materials, authorized other ancillary reagents and authorized
materials required to use your product are not permitted. 6 Traceability refers to tracing analytical sensitivity/reactivity back to an FDA-recommended reference material.
Page 7 – Myoung Shin Kim, LabGenomics Co., Ltd.
W. Authorized laboratories that receive your product will notify the relevant public health
authorities of their intent to run your product prior to initiating testing.
X. Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
Y. Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: CDRH-EUAReporting@
fda.hhs.gov) and you (via email: COVID-19. TechnicalSupport@labgenomics.com) any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
Z. All laboratory personnel using your product must be appropriately trained in RT-PCR
techniques and use appropriate laboratory and personal protective equipment when
handling this kit, and use your product in accordance with the authorized labeling.
LabGenomics Co., Ltd. (You), Authorized Distributors and Authorized Laboratories
AA. You, authorized distributors, and authorized laboratories using your product will
ensure that any records associated with this EUA are maintained until otherwise notified
by FDA. Such records will be made available to FDA for inspection upon request.
Conditions Related to Printed Materials, Advertising and Promotion
BB. All descriptive printed matter, including advertising and promotional materials
relating to the use of your product shall be consistent with the Fact Sheets and authorized labeling, as well as the terms set forth in this EUA and the applicable requirements set forth in the Act and FDA regulations.
CC. No descriptive printed matter, including advertising or promotional materials
relating to the use of your product may represent or suggest that this test is safe or
effective for the detection of SARS-CoV-2.
DD. All descriptive printed matter, including advertising and promotional materials
relating to the use of your product shall clearly and conspicuously state that:
* This test has not been FDA cleared or approved;
* This test has been authorized by FDA under an EUA for use by authorized
laboratories;
* This test has been authorized only for the detection of nucleic acid from SARSCoV-
2, not for any other viruses or pathogens; and,
Page 8 – Myoung Shin Kim, LabGenomics Co., Ltd.
* This test is only authorized for the duration of the declaration that circumstances
exist justifying the authorization of emergency use of in vitro diagnostics for
detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21
U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked
sooner. The emergency use of your product as described in this letter of authorization must comply with the conditions and all other terms of this authorization.
V. Duration of Authorization This EUA will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 is terminated under Section 564(b)(2) of the Act or the EUA is revoked under Section 564(g) of the Act.
Sincerely,
____________________________
RADM Denise M. Hinton
Chief Scientist
Food and Drug Administration
Enclosures
Biolidics Covid-19 Antibody Test

Biolidics Limited manufactures a highly sensitive, low-cost, nearinstant Covid-19 antibody test kit. The company’s test units can detect the presence of novel coronavirus antibodies (2019-nCoVIgG/IgM) in the field in as little as 10 minutes without the use of a lab or special equipment. The units are already in mass production and have been deployed in China. Biolidics is a subsidiary of Clearbridge Health Limited, a publicly-traded Singapore-based medical diagnostics corporation.
The test operates in a manner similar to a pregnancy test. A blood sample is placed on a pad in a cartridge and diffuses upward via capillary force, passing over embedded antigen strips. If the virus is detected, certain lines will appear, depending on the phase of the coronavirus infection in the body (preliminary to latent). Unlike a pregnancy test, however, the Biolidics cartridge uses blood samples. The unit displays a control line indicating a successful test. If additional lines appear, this indicates the presence of IgG and/or IgM antibidoes, signaling that the subject is infected with Covid-19.
The test currently has FDA “Policy D” approval and is awaiting the highly sought-after “Policy C” approval, which includes full FDA endorsement. The test already has Level 3 Sino-FDA approval in China, is approved in Singapore, and has EU approval. The Biolidics product has undergone extensive testing in China.
In the US, NYU-Langone, Harvard / Mass General, and George Washington University Hospital have tested the product. Recent tests at NYU-Langone have yielded 95.83% sensitivity and 93.55% specificity.
The kits are shipped in boxes of 50.
Price: Quote
Origin: Mfg:China Distrib: Singapore
Lead time: 7 days
Shipping: FOB Englewood, CO
Production: 2 mm tests / mo (500,000 / mo to US)
BIOLIDICS LIMITED
(Company Registration Number: 200913076M)
_________________________________________________________________________________
NOTIFICATION TO THE U.S. FOOD AND DRUG ADMINISTRATION FOR THE INTENDED
DISTRIBUTION OF BIOLIDICS’ RAPID TEST KITS FOR NOVEL CORONAVIRUS 2019
The board of directors (the “Board”) of Biolidics Limited (the “Company”) is pleased to announce that the Company has completed the notification process for the intended distribution of its rapid test kits for Novel Coronavirus 2019 (the “COVID-19 Rapid Test Kits”) under Section IV.D of the “Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency” (“Policy D”) of the United States of America (“USA”) and it has received an acknowledgement from the U.S. Food and Drug Administration (“FDA”), a federal agency of the United States Department of Health and Human Services, on the notification process on 9 April 2020 (Singapore time).
Under Policy D, which applies to developers of serology tests that identify antibodies (e.g., IgM, IgG) to SARS-CoV-2 (the virus which causes the disease, the Novel Coronavirus 2019) from clinical specimens, the Company’s COVID-19 Rapid Test Kits are only for use by clinical laboratories or healthcare workers for point-of-care testing and not for at home testing. The Company is also required to provide information along the lines of the following in the test reports:
– The test has not been reviewed by the FDA;
– Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals;
– Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status; and
– Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
The Company wish to highlight that its COVID-19 Rapid Test Kits have not been granted an Emergency Use Authorization by the FDA and the Company is required to complete the listing of its COVID-19 Rapid Test Kits before they can be distributed, marketed and sold to clinical laboratories and healthcare workers for point-of-care testing (the “Listing”). The Company is currently in the process of completing the Listing.
More details about Policy D can be found here: https://www.fda.gov/media/135659/download
The Company will make the appropriate announcement(s) as and when there is further material development relating to this matter.
BY ORDER OF THE BOARD
Yee Pinh Jeremy
Non-Executive Non-Independent Chairman
13 April 2020
Osang GeneFinder Covid-19 PCR Test Kit

The GeneFinder Covid-19 Plus RealAmp Kit is a PCR test kit
manufactured by Osang Healthcare. It is intended for the
qualitative detection of Coronavirus disease 2019 (COVID-19)
strain SARS-CoV-2, in patients that meet the clinical criteria for
COVID-19 (e.g. fever, cough, shortness of breath) in the lower
respiratory tract or upper respiratory tract.
The kits are approved by the US FDA and the EU. Trials on the
kits have yielded sensitivity and specificity in excess of 99%.
Tests run on the Real-time PCR CFX96TM (Bio-Rad), Applied
BiosystemsTM 7500 Real-time PCR Instrument system (Thermo
Fisher Scientific), or Applied BiosystemsTM 7500 Fast Real-time
PCR Instrument system (Thermo Fisher Scientific)
Each kit contains sufficient supplies for 100 tests.
Price: Quote
Origin: Mfg: Republic of Korea
Lead time: 21 days
Shipping: FOB Republic of Korea
Production: 10 mm tests / mo (500,000 /mo to US)
April 18, 2020
David Jack
Strategic Advisor
SBG Distribution, LLC
77 Searing Ave.
Mineola, NY 11501
Device: GeneFinder COVID-19 Plus RealAmp Kit
Company: OSANG Healthcare
Indication: Qualitative detection of SARS-CoV-2 nucleic acids in
nasopharyngeal, oropharyngeal, nasal, and mid- turbinate nasal swab specimens, bronchoalveolar lavage fluid (BAL),
and sputum from individuals who are suspected of COVID-19 by their healthcare provider. Emergency use of this test is limited to authorized laboratories.
Authorized Laboratories: Laboratories certified under the Clinical Laboratory
Improvement Amendments of 1988 (CLIA), 42 USC
§263a, to perform high complexity tests.
Dear Mr. Jack:
This letter is in response to your1 request that the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for emergency use of your product,2 pursuant to Section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. §360bbb-3).
On February 4, 2020, pursuant to Section 564(b)(1)(C) of the Act, the Secretary of the
Department of Health and Human Services (HHS) determined that there is a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad, and that involves the virus that causes COVID-19. Pursuant to Section 564 of the Act, and on the basis of such determination, the Secretary of HHS then declared that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of the virus that causes COVID-19 subject to the terms of any authorization issued under Section 564(a) of the Act.3
__________________________________________________
1 For ease of reference, this letter will use the term “you” and related terms to refer to OSANG Healthcare.
2 For ease of reference, this letter will use the term “your product” to refer to the GeneFinder COVID-19 Plus RealAmp Kit used for the indication identified above.
3 U.S. Department of Health and Human Services, Determination of a Public Health Emergency and Declaration that Circumstances Exist Justifying Authorizations Pursuant to Section 564(b) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3. 85 FR 7316 (February 7, 2020).
Page 2 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
Having concluded that the criteria for issuance of this authorization under Section 564(c) of the Act are met, I am authorizing the emergency use of your product, described in the Scope of Authorization Section of this letter (Section II), subject to the terms of this authorization.
I. Criteria for Issuance of Authorization
I have concluded that the emergency use of your product meets the criteria for issuance of an authorization under Section 564(c) of the Act, because I have concluded that:
1. The SARS-CoV-2 can cause a serious or life-threatening disease or condition,
including severe respiratory illness, to humans infected by this virus;
2. Based on the totality of scientific evidence available to FDA, it is reasonable to believe
that your product may be effective in diagnosing COVID-19, and that the known and
potential benefits of your product when used for diagnosing COVID-19, outweigh the
known and potential risks of your product; and,
3. There is no adequate, approved, and available alternative to the emergency use of your product.4
II. Scope of Authorization
I have concluded, pursuant to Section 564(d)(1) of the Act, that the scope of this authorization is limited to the indication above.
Authorized Product Details
Your product is a qualitative test for the detection of nucleic acid from SARS-CoV-2 in
nasopharyngeal, oropharyngeal, nasal, and mid-turbinate nasal swab specimens, bronchoalveolar
lavage fluid (BAL), and sputum from individuals who are suspected of COVID-19 by their
healthcare provider. The SARS-CoV-2 nucleic acid is generally detectable in upper and lower
respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 nucleic acid; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
To use your product, SARS-CoV-2 nucleic acid is first extracted, isolated and purified from
nasopharyngeal, oropharyngeal, nasal, and mid-turbinate nasal swab specimens, bronchoalveolar lavage fluid (BAL), and sputum. The purified nucleic acid is then reverse transcribed into cDNA followed by PCR amplification and detection using an authorized real-time (RT) PCR instrument. The GeneFinder COVID-19 Plus RealAmp Kit includes the following materials or other authorized materials: COVID-19 Plus Reaction Mixture, COVID-19 Plus Probe Mixture, COVID-19 Plus Positive Control, COVID-19 Plus Negative Control.
__________________________________________________
4 No other criteria of issuance have been prescribed by regulation under Section 564(c)(4) of the Act.
Page 3 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
Your product requires the following control materials, or other authorized control materials, that are processed in the same way as the patient specimens and are required to be included with each batch of specimens tested with your product. All controls listed below must generate expected results in order for a test to be considered valid, as outlined in the Instructions for Use:
· Internal Control – RNase P (RP) control in clinical samples: The RP primer and
probe set is included in each run to test for human RP, which controls for specimen
quality and demonstrates that nucleic acid was generated by the extraction process.
· COVID-19 Plus Positive Control: contains four individual non-infectious DNA
plasmids coding for the RdRp gene, the E gene, the N gene, and the RP gene targeted
by the kit. The positive control is used to monitor for failures of PCR reagents and
reaction conditions.
· COVID-19 Plus Negative Control: Diethyl Pyrocarbonate (DEPC)-treated water used
to monitor non-specific amplification, cross-contamination during experimental
setup, and nucleic acid contamination of reagents.
Your product also requires the use of additional authorized materials and authorized ancillary reagents that are not included with your product and are described in the Instructions for Use. The above described product, when labeled consistently with the labeling authorized by FDA, entitled “GeneFinder COVID-19 Plus RealAmp Kit Instructions for Use” (available at https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-useauthorizations), which may be revised in consultation with, and with concurrence of, the Division of Microbiology Devices (DMD)/Office of Health Technology 7 Office of In Vitro Diagnostics and Radiological Health (OHT7-OIR)/Office of Product Evaluation and Quality (OPEQ)/Center for Devices and Radiological Health (CDRH), is authorized to be distributed to and used by authorized laboratories under this EUA, despite the fact that it does not meet certain requirements otherwise required by applicable federal law.
Your product is authorized to be accompanied by the following product-specific information pertaining to the emergency use, which is required to be made available to healthcare providers and patients:
· Fact Sheet for Healthcare Providers: GeneFinder COVID-19 Plus RealAmp Kit
· Fact Sheet for Patients: GeneFinder COVID-19 Plus RealAmp Kit
I have concluded, pursuant to Section 564(d)(2) of the Act, that it is reasonable to believe that the known and potential benefits of your authorized product, when used for the qualitative detection of SARS-CoV-2 and used consistently with the Scope of Authorization of this letter (Section II), outweigh the known and potential risks of your product.
I have concluded, pursuant to Section 564(d)(3) of the Act, based on the totality of scientific evidence available to FDA, that it is reasonable to believe that your product may be effective for the indication above, when used consistently with the Scope of Authorization of this letter (Section II), pursuant to Section 564(c)(2)(A) of the Act.
FDA has reviewed the scientific information available to FDA, including the information
Page 4 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
supporting the conclusions described in Section I above, and concludes that your product (as described in the Scope of Authorization of this letter (Section II)) meets the criteria set forth in Section 564(c) of the Act concerning safety and potential effectiveness.
The emergency use of your product under this EUA must be consistent with, and may not exceed, the terms of this letter, including the Scope of Authorization (Section II) and the Conditions of Authorization (Section IV). Subject to the terms of this EUA and under the circumstances set forth in the Secretary of HHS’s determination under Section 564(b)(1)(C) described above and the Secretary of HHS’s corresponding declaration under Section 564(b)(1), your product is authorized for the indication above.
This EUA will cease to be effective when the HHS declaration that circumstances exist to justify the EUA is terminated under Section 564(b)(2) of the Act or when the EUA is revoked under Section 564(g) of the Act.
III. Waiver of Certain Requirements
I am waiving the following requirements for your product during the duration of this EUA:
· Current good manufacturing practice requirements, including the quality system
requirements under 21 CFR Part 820 with respect to the design, manufacture,
packaging, labeling, storage, and distribution of your product.
IV. Conditions of Authorization
Pursuant to Section 564(e) of the Act, I am establishing the following conditions on this
authorization:
OSANG Healthcare (You) and Authorized Distributor(s)5
A. Your product must comply with the following labeling requirements under FDAregulations: the intended use statement (21 CFR 809.10(a)(2), (b)(2)); adequatedirections for use (21 U.S.C. 352(f)), (21 CFR 809.10(b)(5), (7), and (8)); any appropriate limitations on the use of the device including information required under 21 CFR 809.10(a)(4); and any available information regarding performance of the device, including requirements under 21 CFR 809.10(b)(12).
B. You and authorized distributor(s) will make your product available with the authorized
labeling to authorized laboratories. You may request changes to the authorized
labeling. Such requests will be made in consultation with, and require concurrence of,
DMD/OHT7-OIR/OPEQ/CDRH.
C. You and authorized distributor(s) will provide to authorized laboratories the Fact Sheet
__________________________________________________
5 “Authorized Distributor(s)” are identified by you, OSANG Healthcare, in your EUA submission as an entity allowed to distribute your device.
Page 5 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
for Healthcare Providers and the authorized Fact Sheet for Patients. You may request
changes to the authorized Fact Sheets. Such requests will be made in consultation
with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
D. You and authorized distributor(s) will make available on your website(s) the Fact Sheet for Healthcare Providers and the Fact Sheet for Patients.
E. You and authorized distributor(s) will inform authorized laboratories and relevant
public health authorities of this EUA, including the terms and conditions herein, and
any updates made to your product, authorized labeling and authorized Fact Sheets.
F. Through a process of inventory control, you and authorized distributor(s) will maintain
records of the authorized laboratories to which they distribute the test and number of tests they distribute.
G. You and authorized distributor(s) will collect information on the performance of your
product. You will report to FDA any suspected occurrence of false positive and false
negative results and significant deviations from the established performance
characteristics of the product of which you become aware.
H. You and authorized distributor(s) are authorized to make available additional
information relating to the emergency use of your product that is consistent with, and
does not exceed, the terms of this letter of authorization.
OSANG Healthcare (You)
I. You will notify FDA of any authorized distributor(s) of your product, including the
name, address, and phone number of any authorized distributor(s).
J. You will provide authorized distributor(s) with a copy of this EUA and communicate to
authorized distributor(s) any subsequent amendments that might be made to this EUA
and its authorized accompanying materials (e.g., Fact Sheets).
K. You may request changes to the Scope of Authorization (Section II in this letter) of your
product. Such requests will be made in consultation with DMD/OHT7-
OIR/OPEQ/CDRH, and require concurrence of, Office of Counterterrorism and
Emerging Threats (OCET)/Office of the Chief Scientist (OCS)/Office of the
Commissioner (OC) and DMD/OHT7-OIR/OPEQ/CDRH.
L. You may request the addition of other instruments and associated software for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
M. You may request the addition of other extraction methods for use with your product. Such requests will be made in consultation with, and require concurrence of,
DMD/OHT7-OIR/OPEQ/CDRH.
Page 6 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
N. You may request the addition of other specimen types for use with your product. Such
requests will be made in consultation with, and require concurrence of, DMD/OHT7-
OIR/OPEQ/CDRH.
O. You may request the addition and/or substitution of primers or probes for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
P. You may request the addition and/or substitution of control materials for use with your product. Such requests will be made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
Q. You may request the addition and/or substitution of other ancillary reagents and
materials for use with your product. Such requests will be made in consultation with,
and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
R. You will evaluate the analytical limit of detection and assess traceability6 of your
product with any FDA-recommended reference material(s). After submission to FDA
and DMD/OHT7-OIR/OPEQ/CDRH’s review of and concurrence with the data, You
will update labeling to reflect the additional testing. Such labeling updates will be
made in consultation with, and require concurrence of, DMD/OHT7-OIR/OPEQ/CDRH.
S. You will track adverse events, including any occurrence of false results and report to
FDA under 21 CFR Part 803.
Authorized Laboratories
T. Authorized laboratories using your product will include with test result reports, all
authorized Fact Sheets. Under exigent circumstances, other appropriate methods for
disseminating these Fact Sheets may be used, which may include mass media.
U. Authorized laboratories using your product will use your product as outlined in the
Instructions for Use. Deviations from the authorized procedures, including the
authorized instruments, authorized extraction methods, authorized clinical specimen
types, authorized control materials, authorized other ancillary reagents and authorized
materials required to use your product are not permitted.
V. Authorized laboratories that receive your product will notify the relevant public health
authorities of their intent to run your product prior to initiating testing.
W. Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
_________________________________________
6 Traceability refers to tracing analytical sensitivity/reactivity back to an FDA-recommended reference material.
Page 7 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
X. Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: CDRH-EUAReporting@
fda.hhs.gov) and you (sales@osanghc.com) any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
Y. All laboratory personnel using your product must be appropriately trained in RT-PCR
techniques and use appropriate laboratory and personal protective equipment when
handling this kit, and use your product in accordance with the authorized labeling.
OSANG Healthcare (You), Authorized Distributors and Authorized Laboratories
Z. You, authorized distributors, and authorized laboratories using your product will ensure that any records associated with this EUA are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
Conditions Related to Advertising and Promotion
AA. All advertising and promotional descriptive printed matter relating to the use of your product shall be consistent with the Fact Sheets and authorized labeling, as well as the terms set forth in this EUA and the applicable requirements set forth in the Act and FDA regulations.
BB. All advertising and promotional descriptive printed matter relating to the use of
your product shall clearly and conspicuously state that:
· This test has not been FDA cleared or approved;
· This test has been authorized by FDA under an EUA for use by authorized
laboratories;
· This test has been authorized only for the detection of nucleic acid from SARSCoV-
2, not for any other viruses or pathogens; and,
· This test is only authorized for the duration of the declaration that circumstances
exist justifying the authorization of emergency use of in vitro diagnostics for
detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21
U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked
sooner.
No advertising or promotional descriptive printed matter relating to the use of your product may represent or suggest that this test is safe or effective for the detection of SARS-CoV-2.
The emergency use of your product as described in this letter of authorization must comply with the conditions and all other terms of this authorization.
Page 8 – David Jack, SBG Distribution, LLC, on behalf of OSANG Healthcare
V. Duration of Authorization
This EUA will be effective until the declaration that circumstances exist justifying the
authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 is terminated under Section 564(b)(2) of the Act or the EUA is revoked under Section 564(g) of the Act.
Sincerely,
____________________________
RADM Denise M. Hinton
Chief Scientist
Food and Drug Administration
Cc: OSANG Healthcare
Enclosures
Feature:
* Lightweight gowns offer comfort and breathability for less critical areas.
* Different size for all ages.
* Spun bond multi-layer fabric material.
* Latex free.
* Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations.
*Available in AAMI PB70:2003 Level 1 and Level 2
Price: Quote (South Korean trading company is monitoring quality control and output on the ground at the factory in China).
Origin: Mfg:China, Distrib: Singapore
MOQ: 10,000
Lead time: 2 Weeks
Shipping: FOB Destination in U.S.
Material: PP Non-woven fabric
Gram Weight: 20 gsm
Color: Yellow
Neck Rope: 35 cm
Waist Rope: 85-90 cm
Sizing: Standard Size (Customized size available)
AAMI: AAMI PB70: 2003 Level 1
Remarks: Long sleeve and elastic cuff 115 X 137 cm Non-Sterile





Price: Quote (South Korean trading company is monitoring quality control and output on the ground at the factory in China).
Origin: Mfg:China, Distrib: Singapore
MOQ: 10,000
Lead time: 2 Weeks
Shipping: FOB Destination in the U.S.
Material: PP Non-woven fabric
Gram Weight: 40 gsm
Color: Blue
Neck Rope: 35 cm
Waist Rope: 85-90 cm
Sizing: Standard Size (Customized size available)
AAMI: AAMI PB70: 2003 Level 2
Remarks: Long sleeve and elastic cuff 115 X 137 cm Non-Sterile
| ** SMS non woven fabric: |
| Spunbond + Meltblown + |
| Spunbond Nonwovens, which is |
| made of Spunbond non- woven |
| fabric + meltblown non-woven |
| fabric + spunbond non-woven |
| three-layer network of hot-rolled. |
Feature:
* Different size for all ages.
* Spun bond multi-layer fabric material.
* Latex free.
* Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk
patient isolation situations.
* Available in AAMI PB70:2003 Level 1 and Level 2




Price: Quote
Origin: Republic of Korea
Sizing: M, L, XL, 2XL
Materials: Polypropylene 50%, Polyethylene 50%
MOQ: 10,000 units
Lead time: 20 days
Shipping: FOB Republic of Korea



Coming Soon
Coming Soon
Coming Soon


Price: Quote
Origin: China
MOQ: 10,000 units
Lead time: 14 days
Shipping: FOB Los Angeles, CA
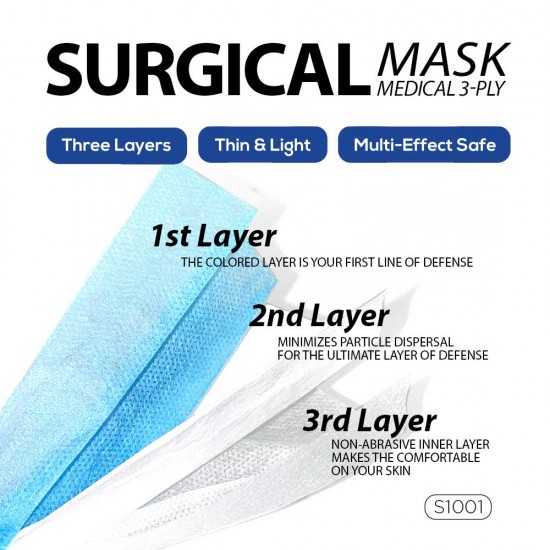
DESCRIPTION
Disposable 3 Ply mask – with a melt-blown polypropylene filtration layer
*Ease of USE – Our masks are disposable and single use. Lightweight mask provides comfort and easy breathing while you’re at work, grocery store, or running necessary errands. Just slip the elastic bands over your ears and conform metal nose guard to create a light seal around your face. Mask should not be reused. Keep the blue-side outside and the nose clip on the upper side.
*GREAT PROTECTION – Our face protective masks are made from non-woven fabric which has increased filtration of fluids in air compared to woven cloth. 3 layers of protection helps filter microbes from entering and exiting mask while not constricting airflow.
Price: Quote
Origin: China
MOQ: 10,000 units
Lead time: 14 days
Shipping: FOB Los Angeles, CA
Description
- Disposable 3 Ply Mask
- All 3 Ply of highest quality materials
- Ease of USE – Our masks are disposable and single use. Lightweight mask provides comfort and easy breathing while you’re at work, grocery store, or running necessary errands. Just slip the elastic bands over your ears and conform metal nose guard to create a light seal around your face. Mask should not be reused. Apply new mask every 2 hours for best protection to ensure moisture from breathe doesn’t dampen mask.
- GREAT PROTECTION – Our face protective masks are made from non-woven fabric which has increased filtration of fluids in air compared to woven cloth. 3 layers of protection helps filter microbes from entering and exiting mask while not constricting airflow.


Price: Quote
Origin: China
MOQ: 10,000 units
Lead time: 3 days
Shipping: FOB Los Angeles, CA
*10 pieces/box
*GB2626 KN95 Standard Mask
Instruction
1. THIS PRODUCT IS USED FOR PROTECTION AGAINST PM2.5, FLOUR, POLLEN, CLEANING, TEXTILE, PHYSICAL GRINDING, WOODWORKING, CONSTRUCTION SITE, PHYSICAL CRUSHING AND OTHER NON-OILY PARTICLES.
2. THIS PRODUCT IS NOT SUITABLE FOR THE ENVIRONMENT WHERE OXYGEN CONCENTRATION IS LOWER THAN 19.5%. IN TOXIC GAS, HARMFUL VAPOR, OIL MIST ENVIRONMENT. RADIOACTIVE MATERIAL POLLUTION AREA, DIOXIN EMISSION RISK AREA.
3. STORAGE REQUIREMENTS: STORE IN A VENTILATED, DARK, DRY ENVIRONMENT, AWAY FROM FIRE AND POLLUTION. THE STORAGE TEMPERATURE IS -20°C -38°C, AND THE STORAGE TEMPERATURE IS LESS THAN 80%. THE STORAGE PERIOD IS UNOPENED FOR 5 YEARS FROM THE PRODUCTION DATE.
4. PRECAUTIONS: DO NOT REMOVE AND WASH THE MASK TO AVOID DAMAGING THE FILTER MATERIAL. WHEN THE MASK IS IN CLOSE CONTACT WITH THE FACE, THE PROTECTIVE EFFECT WILL BE AFFECTED. IF YOU FEEL SIGNIFICANTLY SUFFOCATED, LEAVE THE WORK AREA IMMEDIATELY AND CHANGE YOUR MASK.
5-layer Filtration Design
THIS KN95 FACE MASKS ARE VACUUM STERILIZED, THEIR NON-WOVEN FABRICS PROVIDE ADDITIONAL PROTECTION AND INSULATION AS THEY ESPECIALLY ACT AS FILTERS AND BACTERIAL BARRIERS.
KN95 MASK IS A REAL BREATHING MASK/RESPIRATOR, IT CAN FILTER PARTICLES IN THE AIR VERY EFFECTIVELY.
IT CAN BE USED FOR 3-5 TIMES NORMALLY


Price: Quote 3.38 Fl Oz, 8.0 FL Oz & 16.9 FL Oz. FOB Republic of Korea
Origin: Republic of Korea
MOQ: 50,000 units
Lead time: 10 days
Shipping: FOB Republic of Korea
70% Ethanol

List of Ingredients
Ethanot Purified Water Glycerin Carbomers Trolamine Aloe Extract (09) Parsley Extract Orange Oil Limonene


Price: Quote, pack of 10 sheets
Origin: Republic of Korea
MOQ: 200,000 units
Lead time: 3 Weeks
Shipping: FOB Republic of Korea
Ingredients: Water, Alcohol, Sodium benzoate, Methylpropanediol, Caprylyl Glycol, EDTA-2NA, Ethylhexylglycerin, Octyldodeceth-16
Allguard Antibacterial Wipes are sold in resealable packets each containing 10 wipes. The wipes are appropriate for cleaning hands, objects and surfaces.

Thermometers Menu


Price: Quote
Origin: China
MOQ: 200 units
Lead time: 2-3 Weeks
Shipping: FOB Los Angeles, CA
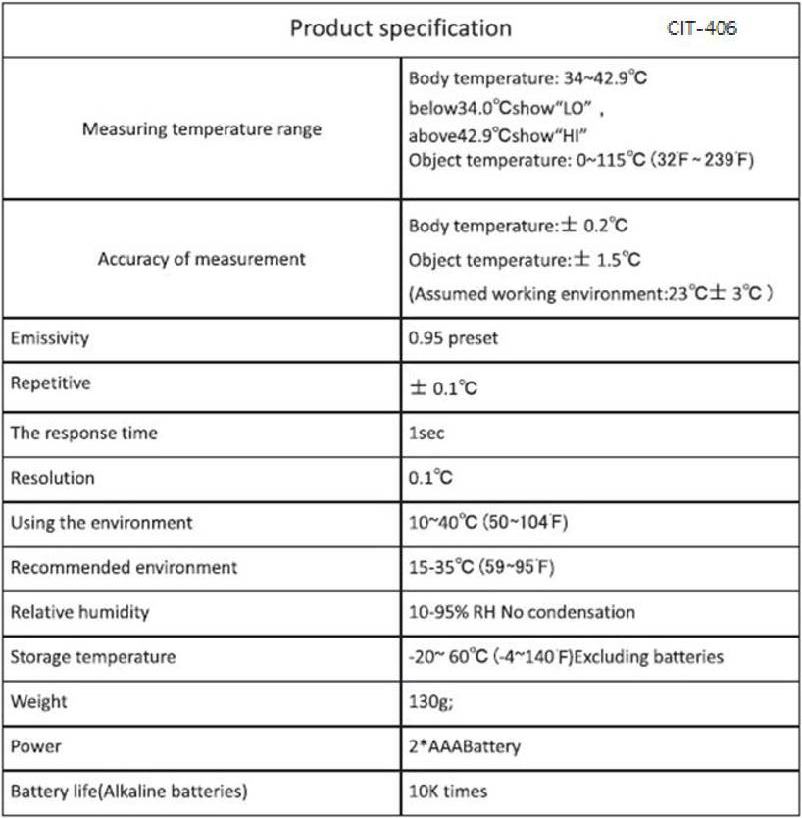



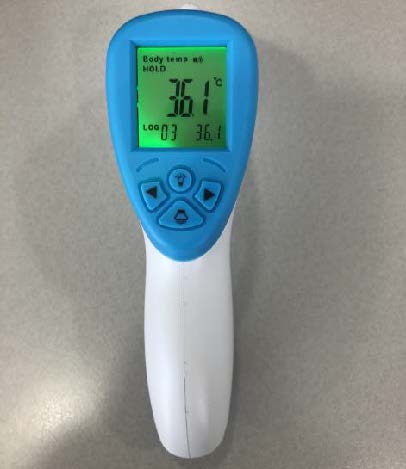

Price: Quote
Origin: China
MOQ: 200 units
Lead time: 2-3 Weeks
Shipping: FOB Los Angeles, CA